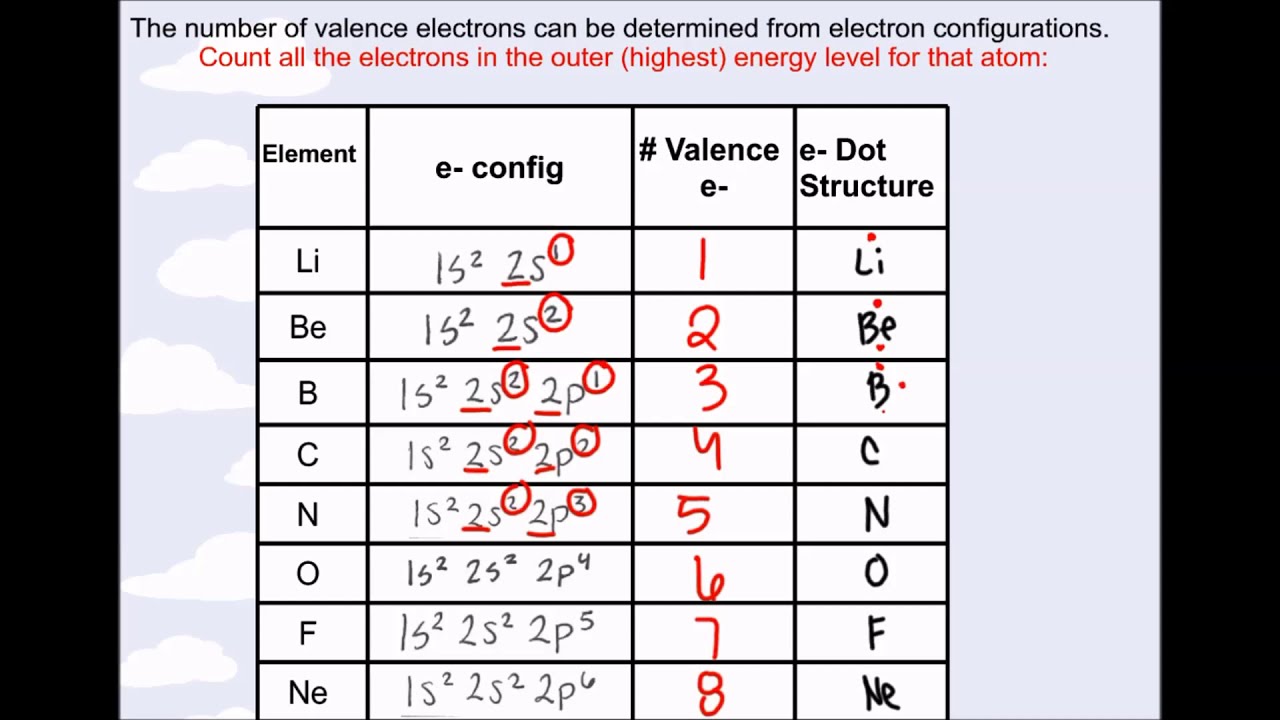
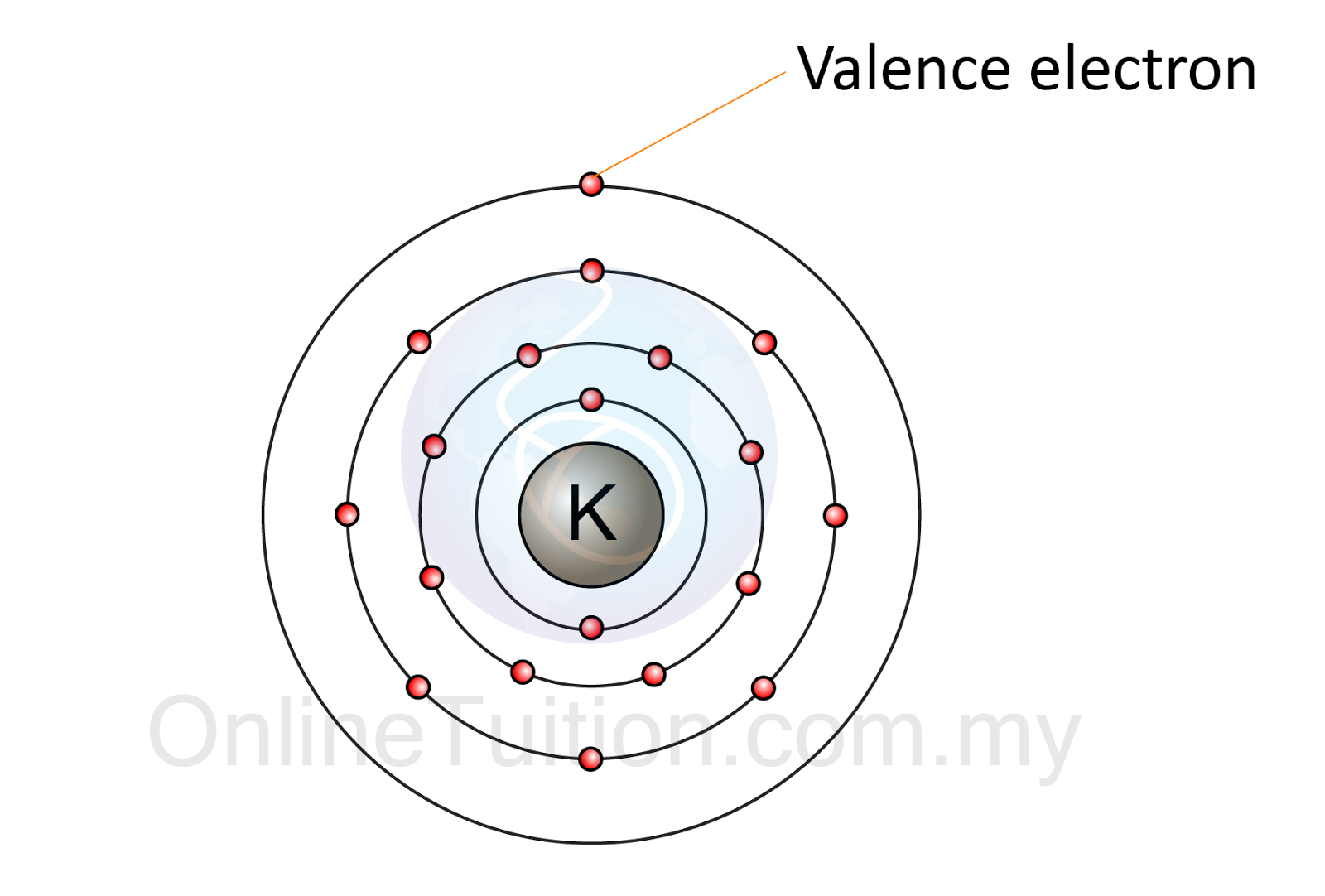
Electron atom valence arrangement number chemistry example sodium protons same find neutrons has chemical elements properties determining Electrons valence number periodic table find elements numbers element where their left right atomic order they located protons ascending contain Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry
Valence Electrons & Electron Configurations - YouTube
Electron arrangement in atom
Valence electrons elements valency electronic electron periodic table shell structure has period notes number many configuration right atomic atom representative
Valence electrons periodic shell recognizing chemicalValence electrons — definition & importance Periodic electron valence electrons unpaired socratic representativePeriodic table compounds chemistry ionic bonds valence covalent each ions element elements electron family lewis molecular symbols dot has ch150.
Valence periodic electrons their worksheets handouts sciencenotesValence electrons / what are valency and valence electrons ascolgyan Valence electronsValence electrons ppt powerpoint presentation level group examples energy elements same.

Periodic table valence chart
Valence electrons electron atom elektron valency atomic orbital nucleus outer element valensi outermost structure molecule bonding within scienceabc shells atomsWhat groups on the periodic table have four unpaired valence electrons Which element could provide one atom to make an ionic bond withValence electrons periodic configuration column.
Valence electrons & electron configurationsElectrons valence many know element ppt does powerpoint presentation do group Valence electrons tin electron socratic metals reactants limiting alkali valance given willCh150: chapter 4 – covalent bonds and molecular compounds – chemistry.

Valence electrons elements group has metals transition which block halogens 17 15 helium noble gases only except sliderbase
What is the number of valence electrons in tin?Electron valence electrons periodic atom nitrogen bond ionic Compounds chemistry bonds covalent ionic valence periodic table element ions each electron molecular family symbols dot column configurations electrons formValence electrons halogens metals noble presentation alkaline gases shell.
Mr. gortney's 8th grade science class / class notes and topicsWhat are valence electrons? definition and periodic table Valence electrons electron configurationsValence electrons.

Atom parts ppt valence electrons powerpoint presentation
Valence electrons .
.